MAICO ERO•SCAN® OAE Screener
| ITEM# | PRODUCT NAME | AVAILABILITY | PRICE | QTY/ACTIONS |
|---|---|---|---|---|
| 1006087 | MAICO ERO•SCAN® OAE Screener with Sessions Software |
In Stock |
$4,628.99
|
|
| 1006088 | MAICO ERO•SCAN® OAE Screener with Sessions Software and Printer |
In Stock |
$4,947.99
|
|
| 51296 | MAICO ERO•SCAN® OAE Screener Printer HM-E200 |
In Stock |
$419.99
|
|
| 51297 | MAICO ERO•SCAN® OAE Screener Thermal Paper for Printer |
In Stock |
$5.79
|
|
| 51269 | MAICO ERO•SCAN® OAE Screener Ear tip Kit |
In Stock |
$96.99
|
|
| 1003236 | MAICO ERO•SCAN® OAE Screener Probe Tubes, 100 ct |
In Stock |
$30.99
|
|
| 1003411 | MAICO ERO•SCAN® Case |
In Stock |
$197.99
|
|
| 1003166 | MAICO ERO•SCAN® Probe Replacement |
In Stock |
$459.99
|
Product Description
Exclusive School Health Trade-In Offer Now Available with Purchase! Click here for details.
Click here for the Trade-In Form from our Service Center.
Hear the difference—schedule your FREE hearing screening consultation today!
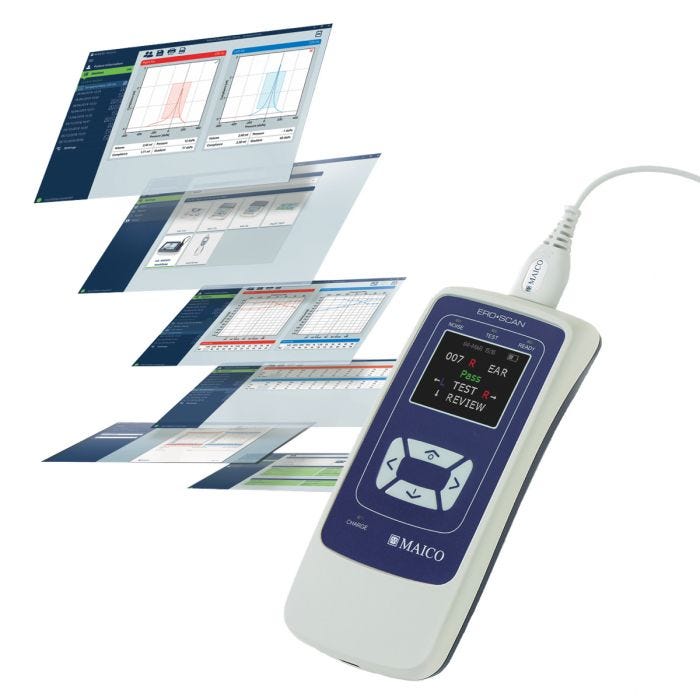
MAICO ERO•SCAN® OAE Hearing Screener is an OAE screener for the early detection of hearing loss. This device quickly screens for hearing loss using objective OAE screening technology of measurement by testing the outer hair cells of the ear and providing a pass/refer response. The smaller design and accessories allow for easy portability and ease of use. Perfect for any age group but recommended for all children aged 3 years and under. OAE screening is also appropriate for children over 3 who are unable to follow the instructions or complete the tasks required for Pure Tone screening.
Features at a glance:
- Fast automatic OAE screening with pass/refer results and graphical displays
- High noise immunity for operation in normal clinical environment
- Small and lightweight ear probe
- Sharp colored OLED display
- Optional wireless printer
- High noise immunity for operation in many environments
- Easy wireless data transfer from ERO•SCAN® device to Sessions Software for L/R mode
MAICO Sessions Software:
Sessions is designed for fast and intuitive operation, allowing you to stay focused on the student. The simple navigation and modern GUI design lets you grasp key details at a glance to explain them clearly. Allows users to print to a PDF or paper via a PC.
- One click exports as PDF or XML
- Standalone or database integration
- Simple and modern user interface
- Icon-based, intuitive navigation
- No training required
- Customizable reports
Explore our School Health YouTube channel to discover a wide array of informative videos showcasing the features and benefits of the MAICO ERO•SCAN® OAE Hearing Screener. Gain valuable insights and tips to make the most of this innovative tool!
Specifications
| Technical | The digital signal processor in the instrument generates two pure tones (f1 and f2) through a digital-to-analog converter. These tones are presented to the ear via speaker tubes located in the probe. A microphone in the probe measures the sound in the ear canal and transmits the signal to the analog-to-digital converter. The digital signal processor then filters the signal into narrow frequency bands, and detects any emissions present. The level of these emissions can be compared with the average level of the noise in adjacent frequency bands. An emission is judged to be present when the level in the emission band is 5 db or more above the level in adjacent bands. (The actual pass-fail criterion used in the EroScan instrument uses a more sophisticated statistical test). |
|---|---|
| Warranty | The MAICO ERO•SCAN® is guaranteed for at least one year. This warranty is extended to the original purchaser of the device by MAICO through the distributor (School Health) from whom it was purchased and covers defects in material and workmanship for a period of at least one year from date of delivery of the device to the original purchaser. |
| Brand | MAICO |
| Contents | MAICO ERO•SCAN OAE® Hearing Screener includes: Carry case, USB cable, wall charger, 120 ear tips, 100 probe tubes, and probe tube remover. MAICO Sessions software for PC transfer/print, user guide and quick start guide. Ear Tip Kit (51269) includes: (10) preemie, (15) 3-5mm flanged, (15) 4-7mm flanged, (10) 7mm mushroom, (10) 8mm mushroom, (10) 9mm mushroom, (10) 10mm mushroom, (10) 11mm mushroom, (10) 12mm mushroom, (10) 13mm mushroom, (10) 14mm mushroom, (10) 15mm mushroom, (1) ear tip remover and (100) probe tubes. |
| Assembly Required | No |
| Calibration | Annual calibration is crucial for preserving the accuracy of your OAE. Neglecting this vital maintenance step can result in faulty equipment, leading to inaccurate measurements and potentially incorrect diagnoses. Visit the School Health Service Center for Expert Calibration Services!
|
| Training | Comprehensive training includes: The MAICO ERO•SCAN® OAE Hearing Screener comes with in-person or online training tailored to your needs—just give us a call for more details. School Health is proud to be certified and licensed by MAICO as the exclusive provider of training & service for the MAICO ERO•SCAN® OAE Hearing Screener across all states! |
Reviews
Documents
Calibration
| Calibration | Annual calibration is crucial for preserving the accuracy of your OAE. Neglecting this vital maintenance step can result in faulty equipment, leading to inaccurate measurements and potentially incorrect diagnoses. Visit the School Health Service Center for Expert Calibration Services!
|
|---|
Training
| Training | Comprehensive training includes: The MAICO ERO•SCAN® OAE Hearing Screener comes with in-person or online training tailored to your needs—just give us a call for more details. School Health is proud to be certified and licensed by MAICO as the exclusive provider of training & service for the MAICO ERO•SCAN® OAE Hearing Screener across all states! |
|---|
Faq
| Faq | Q: What does a REFER result mean? A: Test results are either PASS or REFER. REFER means that the patient did not pass the test. This could be due to many reasons including ear wax, middle ear fluid, noise, improper test technique or hearing loss. All REFER results should be immediately repeated. If the test result continues to be REFER, the patient should be screened using pure-tone testing and tympanometry. If passing results are not achieved on these tests, then a referral to an audiologist and/or physician should be made. Who Can Be Screened: Q: What ages can be screened with the EroScan? A: Newborn to adult. Q: Can I test patients with pressure equalization (PE) tubes? A: Patients with PE tubes can be tested by bypassing the auto-start function of the unit. This is accomplished by first inserting the EroScan with an appropriate ear tip into the ear canal and obtaining a proper seal. To disable the auto-start, at the main menu select the ear to be tested by holding down the right or left arrow keys for 3 seconds until the green "test" light turns off. Once the key is released, the EroScan will calibrate and test as before. Q: If a patient's ear is impacted with wax can I still screen her? A: No, impacted wax will yield a refer result. In addition, any significant amount of wax can potentially cause a refer result. Q: Can a patient with otitis media still be screened with the EroScan unit? A: Middle ear fluid will yield a refer result. This should then be followed up with pure tone testing and tympanometry so you can determine if there is otitis media based on the flat tympanogram. Maintenance: Q: Does the unit need to be calibrated? A: Equipment is not user repairable. Repairs and battery replacement must be performed by a qualified service representative only. Annual calibration is recommended. Q: How do I clean my EroScan unit? A: The instrument and its accessories may be wiped clean with a damp cloth using a mild antiseptic solution (e.g. cetylcide). Take care not to put excessive pressure on the clear display window or allow any utensil to puncture the display window or keypad. Do not allow any fluid to enter the device. Do not immerse the instrument in fluids or attempt to sterilize the instrument or any or its accessories. Probe tips should be replaced when they become clogged. Replacement probe tips are included with the instrument. Do not attempt to clean the probe tips, they are disposable and must be replaced when they become clogged. |
|---|
Contents
| Contents | MAICO ERO•SCAN OAE® Hearing Screener includes: Carry case, USB cable, wall charger, 120 ear tips, 100 probe tubes, and probe tube remover. MAICO Sessions software for PC transfer/print, user guide and quick start guide. Ear Tip Kit (51269) includes: (10) preemie, (15) 3-5mm flanged, (15) 4-7mm flanged, (10) 7mm mushroom, (10) 8mm mushroom, (10) 9mm mushroom, (10) 10mm mushroom, (10) 11mm mushroom, (10) 12mm mushroom, (10) 13mm mushroom, (10) 14mm mushroom, (10) 15mm mushroom, (1) ear tip remover and (100) probe tubes. |
|---|
Warnings
| Choking Hazard | No |
|---|---|
| Sterile | No |